
BY ACOG FOR ALL WOMEN WHO ARE CONSIDERING PREGNANCY
Many genetic disorders resulting from gene abnormalities don’t show trait or symptoms of the disease but still have mutations within the gene are called carrier. Melanin can be inherited from parents or de novo mutation ; a mutation present for the first time in family member. If these mutations are passed down to the offspring, there are chances of phenotypic abnormalities.
Carrier screening for genetic mutation helps marriage partners who plan to have children learn of the risks of any latent mutated genes before pregnancy, plan treatments using suitable fertilization technology, as well as the option of gamete donor. This is to lower the risk of genetic disorders from being inherited by the offspring.
Severe genetic disorders commonly found are Spinal Muscular Atrophy (SMA), Fragile-X syndrome, Hemoglobinopathy, Cystic Fibrosis. The American College of Obstetricians and Gynecologists (ACOG) recommends all women who plan to have children to undergo genetic carrier screening for these four genetic disorders. The characteristics of each disorders are listed below.
1. Spinal Muscular Atrophy (SMA)
Spinal Muscular Atropy is a genetic disorder with a characteristic of muscle weakness that results from dysfuntion of motor neurons. This mutation leads to the loss of signal from the spinal cord to muscles. It is ranked number 2 as the most common genetic disorder following Thalassemia.
CAUSE OF SMA
≥ In a normal human body, there are 2 genes that encode the Survivor Motor Neuron (SMN) genes to control the muscles motor neurons. SMN1 is the main producer of the genes, while SMN2 produces only 10% of the genes. Each gene has 2 copies and is located on chromosome 5q13. SMN is due to deletion or point mutation in SMN1 resulting in a truncated SMN protein which is rapidly degraded in the cell, leading to loss of muscle control, resulting in muscle weakness and the loss of the ability to move.
Nevertheless, SMN2 in the human body resembles a back-up with various number of copies in each person. Reports have shown that a person with homozygous deletion of SMN1 can have no symptoms of SMA. This is because this person has 5 copies of SMN2, even though the production is only 10-20%. The hereditary pattern of SMA is autosomal recessive. (Figure 1) Carriers of this disorder will have 1 copy of SMN1. If each parent has 1 copy of SMN1 (SMA carrier), their child has a 1 in 4 chance of inheriting SMA. Another type of SMA carrier is called a silent carrier. Testing finds 2 copies of SMN1 like in regular case but they are located on the same chromosome. The other chromosome is left with no SMN1 which can result in abnormalities in the offspring as well.
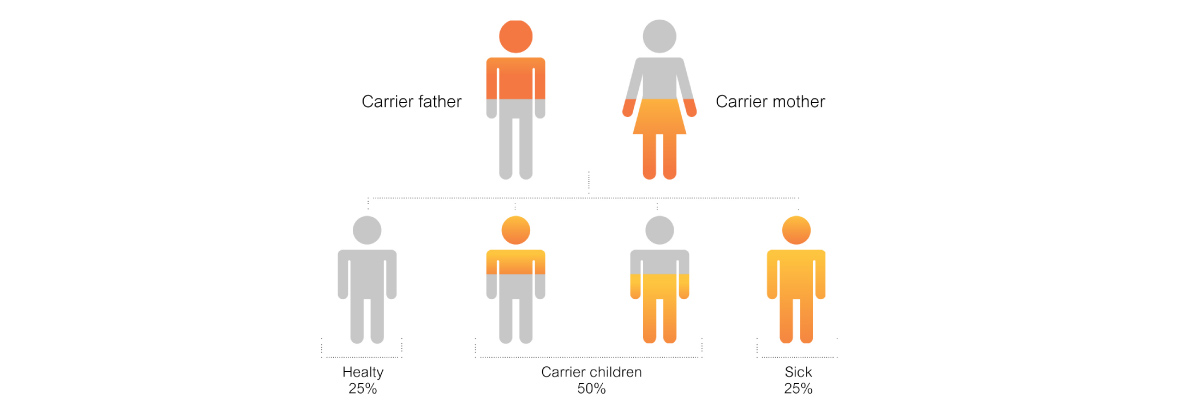
Figure 1 Autosomal recessive inheritance patternIn the case of a family where both the father and the mother are carriers, the chances of having a healthy child are 25%, while the likelihood of having a carrier child are 50%. Finally, there would be a 25% chance of having a sick child.
GENETIC TESTING
≥ Jetanin’s genetic lab offers SMA1 copy number analysis with the TagMan q PCR technique. The inspector can draw 6-10 ml of blood into standard EDTA tube and send to the genetic lab. Results come out within 7 working days.
2. Fragile-X syndrome
The symptom of Fragile-X syndrome is delayedintellectualdevelopment . This syndrome is genetically passed down via chromosome X. The level of intelligence of a person with Fragile-X syndrome is medium to low. He/she exhibits autistic behaviors and attention-deficit/hyperactivity. The intellectual disabilities are more severe in males.
CAUSE OF FRAGILE-X SYNDROME
≥ Fragile-X syndrome results from the mutation of the fragile X mentalretardation-1 (FMR1) gene on Chromosome X at q27.3. The mutation is a tripiet repeat of base Cytosine-Guanine-Guanine (CGG) at the beginning of the FMR1 (5’ UTR). There are 4 types of repeated CGG segment according to the number of repeats
1.) less than 40 repeats, found in normal human.
2.) 41-55 repeats (intermediate), no phenotype, passed down to offspring.
3.) 56-200 repeats, found in carriers, called premutation.
4.) more than 200 repeats, found in people that has the disorder (full mutation).
The prevalence of this disease in males is 1 in 4000 and in female is 1 in 6000-8000. The occurrence of carriers found in male is 1 in 800 and in female is 1 in 100-200.
3. Hemoglobinopathy
Hemoglobins (Hb) are proteins inside red blood cells. They function in binding oxygen. One unit of hemoglobin consists of 4 chains of proteins: 2 chains of α-globin and 2 chains of β-globin Hemoglobinopathy can be divided into 2 types.
1.) make little to no hemoglobin (alpha, beta-thalassemia)
2.) shape abnormalities (Hb variants) from mutation. Even though hemoglobins are being made regularly, they are dysfunctional and have negative effects on the cell such as sickle cell anemia and Hb S. Apart from this, there are mutations that changes the shapes and lower the production (Thalassemia Hb variants) such as Hb constant spring, Hb E, and Hb Paksé. The type of hereditary is autosomal recessive. (Figure 1)
PRIMARY SCREENING AND GENETIC TESTING
≥ Partners who plan to have a child or an expecting wife should receive complete blood cell count as well as testing for Thalassemia (Hb typing). The ratio of each type of Hemoglobin should be examined as initial screening. It is recommended especially in couples who have a Mean Corpuscular Volume lower than 85 fL to undergo genetic testing to screen for the mutated genes.
Because Thalassemia is a genetic condition that is number one most commonly found and can have a very high severity. The mutation can be tested using multiplex PCR. Results can be analyzed by capillary electrophoresis. ThJetanin genetic lab provides the test for alphathalassemia 7 deletions variants that is commonly found in this region. Results take 7-10 days to come out.
4. Cystic Fibrosis
Cystic fibrosis (CF) is a genetic disorder that causes epithelial cell abnormalities of the airways, reproductive system, and gastrointestinal tract, especially in the liver and pancreas. In 2001, it is recommended to test for CF before and during pregnancy in order to lower the risk of the child having respiratory and pancreas abnormalities. CF is most commonly seen in Caucasians at around 1 in 2,500.
CF is caused by the mutation of the cystic fibrosis transmembrane regulator (CFTR) which resides on the chromosome 7. The type of hereditary is autosomal recessive. Recently, more than 1,700 types of mutation are found with 23 types frequently found. Nonetheless, the prevalence of CF is low in Asian population.
Suggestions When Plan on Having a Child and on Genetic Carrier Screening
According to The American College of Obstetricians and Gynecologists (ACOG) suggestions on carrier genetic screening are as follows:
1. Marriage partners should receive a genetic carrier screening before conceiving.
2. If a husband or a wife is tested positive for a carrier, his or her partner should also be tested in order to plan the pregnancy and assess the risks.
3. If both marriage partners tested positive for carriers, a close follow-up prenatal diagnosis is important in order to lower the risk of the infant from inheriting the gene from the mother, father, or both.
4. When tested positive for a carrier of a genetic disorder, one should suggest relatives to also receive a genetic carrier screening as they also hold the risk of being a carrier. It is possible for one to have a carrier gene of a genetic disorder. If one receives a genetic carrier screening and learn of any carrier gene, one can plan effectively and prepare for a pregnancy that is safe for both the mother and the child.
Written by
Sivadatch Chooduang,
Scientist, Genetics Laboratory
Jetanin Hospital
JETANIN Journal Vol.11 No.1
